FDA Says Facebook ‘Likes’ Equals Illegal Medical Advice and Health Claims
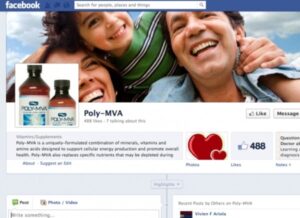
The FDA has sent warning letters to several supplement makers about health claims the companies have been making on social media platforms including Facebook and Twitter, reports NutraIngredients-USA.com.
One of the companies receiving a warning was AMARC Enterprises for allegedly “liking” a comment left by a fan on their Poly-MVA Facebook page stating that the company’s dietary supplement helped keep cancer away without chemo or radiation treatment. This simple act of “liking” the comment is considered an endorsement of using the product as a drug, rather than a supplement, which is unsafe, says the FDA.
AMARC Enterprises also put a link on its Facebook page to an article about using alternative treatments for cancer in children.
The issue of manufacturers “liking” comments left on their Facebook page by fans is a tenuous debate, as proving that the “like” is an endorsement, medical or health claim is difficult to verify. NutraIngredients-USA.com quoted Bethany Kennedy, an associate at the law firm Emord & Associates, on the issue: “Whether or not the FDA will construe a company’s failure to delete from its wall a testimonial from a consumer that makes disease claims as evidence of intended has yet to be seen. As a result…companies will have to expend resources policing consumer comments, possibly unfriending consumers who make disease claims.”
NutraIngredients-USA.com also reports that the FDA has sent at least two letters to M.D.R. Fitness Corp for allegedly tagging certain products on its website to appear when consumers use the site’s search engine to look for conditions such as cancer or diabetes. This implies the company is suggesting the products are intended to diagnose, treat, cure or prevent the illnesses, claims that can only be made by drug manufacturers, says the FDA.
Keep in touch with Jill on Twitter @jilletinger

